Document
About Bella
Contact
Abbreviations
| Abbreviation | Explanation |
|---|---|
| IVI | International Vaccine Institute |
| CRF | Case Report Form |
Assumptions & Constraints
- A.
- B.
- A.
- B.
- C.
Requirements
For the best experience and compatibility, we highly recommend you installing the latest version of Google Chrome.
User roles & Permissions
User roles defined in Bella EDC are grouped based on organizations and represented as follows:
IVI: User Administrator, Study Creator (IVI Data Manager), Study Medical Monitor, Clinical Operations, Biostatistician, Quality Management, Clinical Research Lab
SITE: Principal Investigator, Sub Investigator, Site Coordinator, Site Data Encoder, Site Data Manager
CRO: CRA, Safety Specialist
OTHERS: Auditor, Guest, Coder
*
UA
User Administrator,
SCR
Study Creator,
SMM
Study Medical Monitor,
CO
Clinical Operations,
BST
Biostatistician,
QM
Quality Management,
CLR
Clinical Research Lab,
PI
Principal Investigator,
SI
Sub Investigator,
SCD
Site Coordinator,
SDM
Site Data Manager,
SDE
Site Data Encoder,
CRA
Clinical Research Associate,
SS
Safety Specialist,
ADT
Auditor,
GST
Guest,
CDR
Coder
| Object | Activities | UA | SCR | SMM | CO | BST | QM | CRL | PI | SI | SCD | SDM | SDE | CRA | SS | ADT | GST | CDR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| e-CRF | Entry / Edit | X | X | X | X | X | ||||||||||||
| Inactivate / Reactivate | X | X | ||||||||||||||||
| Export | X | X | X | X | X | |||||||||||||
| Review | X | X | ||||||||||||||||
| SDV | X | X | ||||||||||||||||
| Confirm / e-signature | X | X | ||||||||||||||||
| Lab | Upload | X | ||||||||||||||||
| Download | X | X | X | |||||||||||||||
| Query | Open | X | X | X | X | X | X | X | ||||||||||
| Respond | X | X | X | X | X | |||||||||||||
| Close | X | X | X | X | X | X | X | |||||||||||
| Review | X | X | X | X | X | X | X | X | X | |||||||||
| User | Role assign | X | X | X | X | X | ||||||||||||
| PWD reset | X | X |
How to sign-up/-in
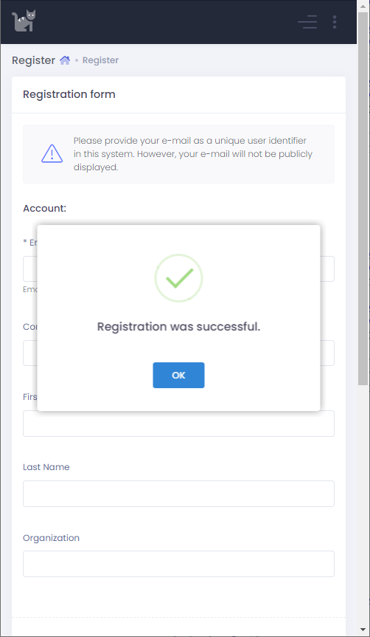
When you successfully submit the data you entered, you will see the message and get an activation email.
How to build a study



